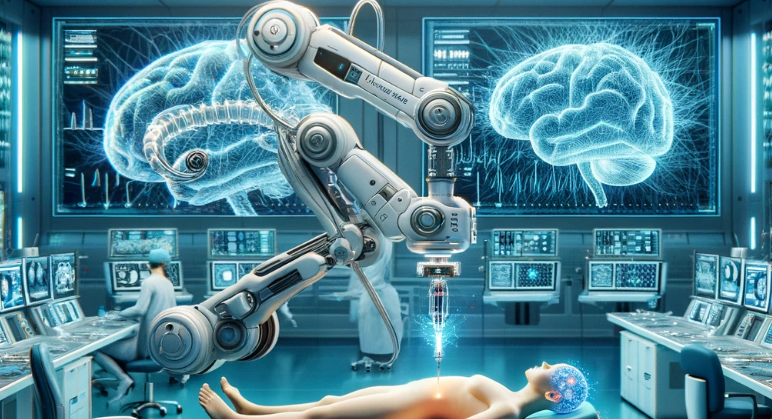
In a move that marks a significant leap forward in the realm of medical science and technology, Neuralink, the brain-child of billionaire visionary Elon Musk, has successfully implanted its first brain chip into a human patient. This momentous event took place on Sunday, with the patient reportedly recovering well from the procedure, according to Musk’s announcement on the social media platform X.
The Promise of Neural Spikes
Musk’s excitement is palpable as he shares the initial results, noting “promising neuron spike detection.” For the uninitiated, neuron spikes are essentially the electrical signals that neurons, the brain’s communication experts, use to transmit information. The ability to detect these spikes is crucial for interpreting brain activity, which could revolutionize how we understand and interact with the human brain.
FDA Green Light and Neuralink’s Vision
This milestone follows last year’s nod from the U.S. Food and Drug Administration (FDA), allowing Neuralink to embark on human trials. This approval was a crucial step for the company, which aims to harness its technology to assist patients suffering from paralysis and various neurological conditions. By September, Neuralink was already gearing up, having received the go-ahead to start recruiting participants for its human trial.
The trial involves a sophisticated robot that surgically implants a brain-computer interface (BCI) in a brain region responsible for movement intention. The ultimate goal? To enable individuals to control digital devices through thought alone. The technology, which Neuralink refers to as “ultra-fine” threads, is designed to capture and transmit brain signals with unprecedented precision.
Introducing Telepathy: The Future of Communication
Musk teases the first product from Neuralink, aptly named Telepathy, hinting at a future where thought-to-communication could become a reality. The ongoing PRIME Study, which focuses on the wireless BCI, aims to assess the safety of both the implant and the surgical procedure involved.
Challenges and Controversies
Despite these advancements, Neuralink has not been without its controversies. Recent reports highlighted the company’s fine for breaching U.S. Department of Transportation regulations on hazardous materials transport. Furthermore, concerns have been raised by lawmakers over the safety of Neuralink’s technology, especially following issues observed in animal trials, such as paralysis and brain swelling. Musk, however, has defended the company’s track record, emphasizing the careful selection of subjects to minimize risk.
Looking Ahead
As Neuralink continues to navigate both breakthroughs and challenges, the world watches with bated breath. The potential of BCI technology to transform lives is immense, offering hope to millions suffering from neurological conditions. Yet, it also brings forth ethical and safety considerations that must be meticulously addressed. As we stand on the cusp of a new era in medical science, the journey of Neuralink serves as a testament to the boundless possibilities that innovation and perseverance can unlock.
Stay up to date on other relevant news in our “News and Politics” section.